New Delhi: An eye drop of an Indian pharmaceutical company has caused a stir in the US. Three people have died in the US due to the use of this eye drop.
The top medical monitoring agency in the US has expressed concern over the possibility of highly drug-resistant bacteria in this eye drop, a news report said.
According to the report, cases of blindness and dozens of infections have been reported in eight people using this eye drop so far.
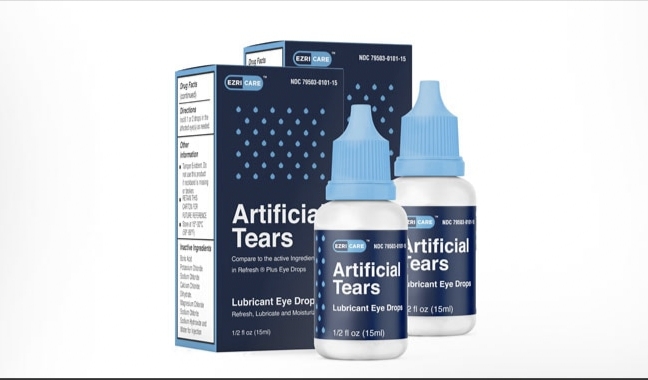
Citing the Centers for Disease Control and Prevention (CDC), news reported that three deaths, eight cases of blindness and dozens of infections have been reported after the use of this eye drop. The eye drop has been developed under the brand name Azricare Artificial Tiers by Chennai-based Global Pharma Healthcare.
The CDC is very concerned that the drug-resistant bacteria found in eye drops imported from India can gain a foothold in the US. Infectious disease experts said that this sten has not been found before in the US. In such a situation, the problem is that it is very difficult to treat it with existing antibiotics in the US.
Global Pharma Healthcare, located about 40 km south of Chennai, stopped producing eye drops linked to the US market in February. It has also voluntarily withdrawn all the remaining lots of EzriCare Artificial Tiers and Delsum Pharma’s Artificial Tiers at the consumer level.
This is the latest case of Indian eye drops after dozens of children died of cough medicine in Gambia and Uzbekistan last year. The US Food and Drug Administration has said that the use of Azricare artificial tears increases the risk of eye infections that can result in blindness or death.